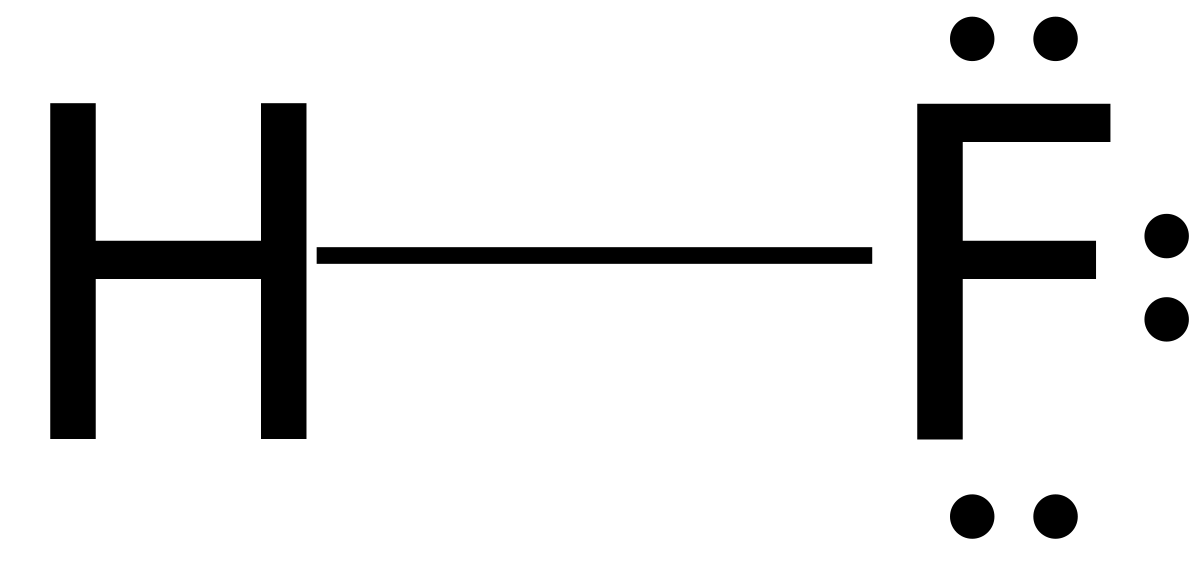Hydrofluoric Acid
Product Details:
| CAS No | 7664-39-3 |
| Grade Standard | Industrial Grade |
| Physical State | Liquid |
| Usage/Application | Catalyst, etchant, and precursor in various industries including pharmaceuticals, electronics, and petrochemicals. |
| HS Code | 28111100 |
| Packaging Details | 200 litres Drum |
| Chemical Formula | HF |
| Purity % | 40%, 48%, 60% |
| Country of Origin | Made in India |
Hydrofluoric Acid Overview:
Hydrofluoric acid (HF) is a highly corrosive and toxic inorganic acid, with the chemical formula HF. It is a colorless solution with a pungent odor and is capable of dissolving various materials, including glass and metals. Hydrofluoric acid is widely used in industrial processes, laboratory applications, and as an etchant in semiconductor manufacturing.
Key Characteristics:
- Highly corrosive liquid
- Colorless solution with a pungent odor
- Capable of dissolving silica-based materials, such as glass
Applications:
- Etchant: Hydrofluoric acid is used in semiconductor manufacturing as an etchant for silicon dioxide (glass) and silicon nitride.
- Glass Etching: It is employed in the glass industry for etching and engraving designs on glass surfaces.
- Metal Pickling: Hydrofluoric acid is utilized for removing oxide and scale from metal surfaces in various industrial processes.
- Chemical Synthesis: It serves as a precursor in the production of fluorine-containing compounds, such as fluorocarbons and fluorinated pharmaceuticals.
- Laboratory Use: Hydrofluoric acid is used in laboratories for chemical analysis, glassware cleaning, and as a reagent in organic synthesis.
Hazards:
- Highly corrosive and toxic.
- Can cause severe burns upon contact with skin, eyes, or mucous membranes.
- Inhalation of vapors can lead to respiratory irritation, coughing, and lung damage.
- Exposure to hydrofluoric acid can result in systemic toxicity and potentially fatal systemic effects if absorbed through the skin.