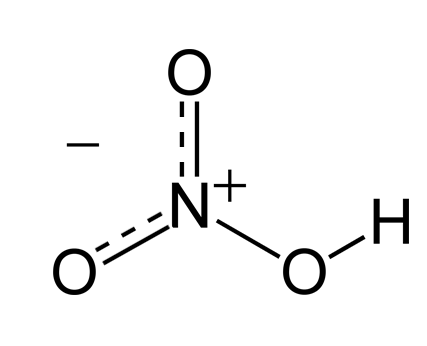
Nitric Acid
Product Details:
| CAS No | 7697-37-2 |
| Grade Standard | Industrial Grade |
| Physical State | Liquid |
| Usage/Application | Widely employed in industries for chemical synthesis, metal processing, and as a reagent in fertilizer production. |
| HS Code | 28080012 |
| Packaging Details | 200 litres Drum |
| Chemical Formula | HNO? |
| Purity % | >60% |
| Country of Origin | Made in India |
Nitric Acid Overview:
Nitric acid (HNO3) is a highly corrosive and strong mineral acid commonly used in various industrial processes, laboratory applications, and as a reagent in chemical synthesis. It is a colorless to yellowish liquid with a sharp, acrid odor and is known for its powerful oxidizing properties.
Key Characteristics:
- Highly corrosive mineral acid
- Colorless to yellowish liquid with a sharp odor
- Strong oxidizing agent
Applications:
- Metal Etching: Nitric acid is used for etching and cleaning metals, such as stainless steel and aluminum, in various industrial processes.
- Fertilizer Production: It is a key ingredient in the production of ammonium nitrate, a widely used fertilizer.
- Explosives Manufacturing: Nitric acid is utilized in the production of explosives, such as TNT (trinitrotoluene) and nitroglycerin.
- Laboratory Reagent: It serves as a reagent in chemical analysis, organic synthesis, and as a nitrating agent.
- Pickling Agent: Nitric acid is used for pickling metals to remove scale, rust, and other impurities.
Hazards:
- Highly corrosive and strong oxidizing agent.
- Can cause severe burns upon contact with skin, eyes, or mucous membranes.
- Inhalation of vapors can lead to respiratory irritation, coughing, and lung damage.
- Exposure to nitric acid fumes may result in pulmonary edema and systemic toxicity.