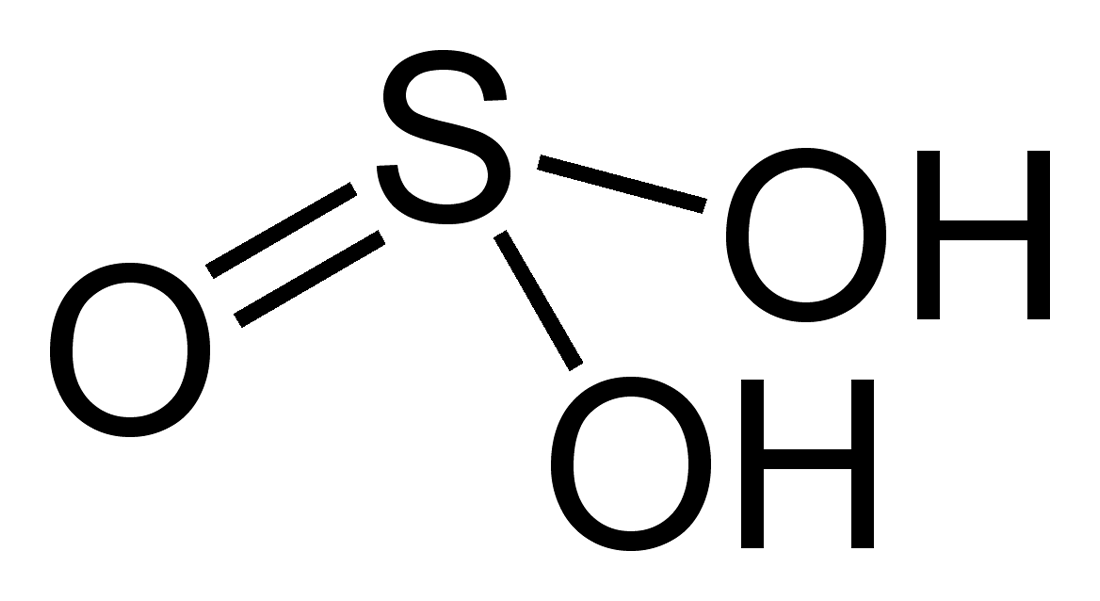
Sulfuric Acid
Product Details:
| CAS No | 7664-93-9 |
| Grade Standard | Industrial Grade |
| Physical State | Liquid |
| Usage/Application | Widely used in industries for chemical synthesis, metal processing, and as an electrolyte in lead-acid batteries. |
| HS Code | 28070010 |
| Packaging Details | 200 litres Drum |
| Chemical Formula | H?SO? |
| Purity % | >95% |
| Country of Origin | Made in India |
?
Sulfuric Acid (H2SO4) Overview:
Sulfuric acid, with the chemical formula H2SO4, is a strong mineral acid known for its corrosive properties and diverse industrial applications. It is colorless to slightly yellow in its pure form and is commonly used in manufacturing, chemical synthesis, and laboratory procedures.
Key Characteristics:
- Strong mineral acid
- Colorless to slightly yellow liquid
- Highly corrosive and reactive
Applications:
- Chemical Manufacturing: Sulfuric acid is a key component in the production of various chemicals, including fertilizers, explosives, detergents, and pharmaceuticals.
- Petroleum Refining: It is used in petroleum refining processes for the purification of petroleum products and as a catalyst in alkylation reactions.
- Battery Manufacturing: Sulfuric acid is used in lead-acid batteries as an electrolyte to facilitate energy storage and discharge.
- Metal Processing: It is employed in metal processing industries for pickling, electroplating, and metal surface treatment.
- Water Treatment: Sulfuric acid is used in water treatment plants for pH adjustment, wastewater treatment, and the removal of heavy metals.
Hazards:
- Highly corrosive and can cause severe burns upon contact with skin, eyes, or mucous membranes.
- Inhalation of vapors may cause irritation to the respiratory system and damage to lung tissue.
- Ingestion of concentrated solutions can lead to severe gastrointestinal injury and internal corrosion.
- Sulfuric acid can react violently with water and other substances, posing fire and explosion risks.
[/read]